OUR PRODUCT
At Frontier Diagnostics, we believe better diagnostics save lives.
We offer state-of-the-art blood tests and AI-powered tools to help patients and doctors monitor cancer for the detection of early relapse, treatment success and personalised care through quantitative drug testing.
SENTINEL qCTC
A minimally invasive blood-sample–based assay designed for the research detection of living circulating tumour cells (CTCs) — providing investigators with real-time data to study tumour biology and dynamics.


WHAT IS IT?
Sentinel qCTC is a research-use-only liquid biopsy assay designed to detect and quantify circulating tumour cells (CTCs) in blood samples. CTCs are intact, living tumour cells that may enter the bloodstream after detaching from a primary tumour or metastatic site. By enabling sensitive measurement of these cells, Sentinel qCTC provides researchers with valuable insights into tumour biology, disease dynamics, and the potential role of CTCs as biomarkers. This technology is currently still intended for research purposes only and is not yet cleared or approved for diagnostic use.
The Test;
- Uses only a small 15 ml EDTA blood sample
- Is non-invasive and repeatable over time
- Is analysed in an ISO 15189-accredited laboratory
- Or as a CE marked medical device (Currently being worked on)
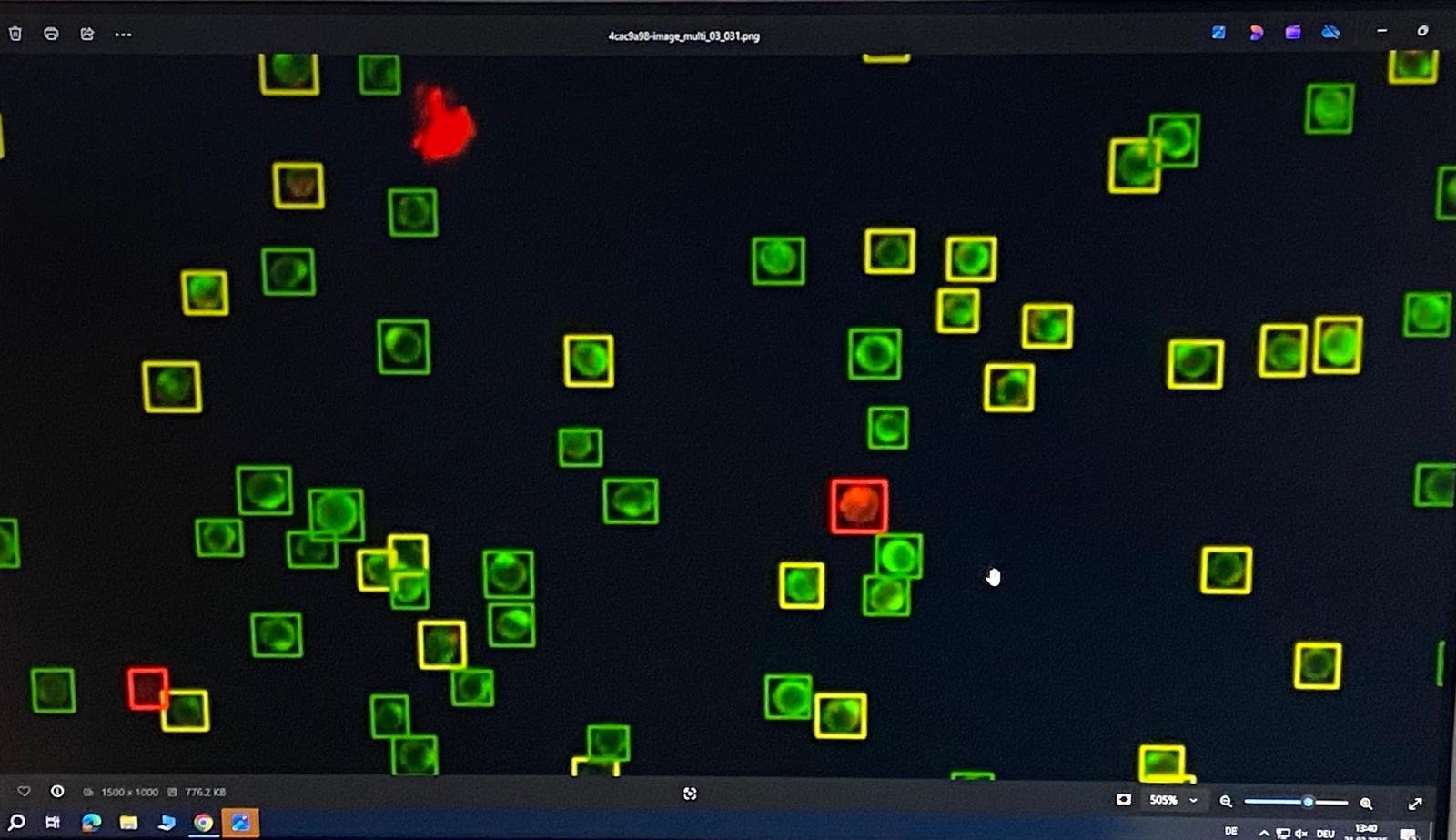
DIAGNOSTIC & MONITORING USES
Sentinel qCTC is a highly sensitive, research use only liquid biopsy assay that detects and enumerates Circulating Tumour Cells (CTCs) in blood samples. These rare, living tumour cells may provide researchers with unique insights into tumour biology, disease progression and treatment response.
As part of ongoing research qCTC is being explored for its potential to:
- Support earlier detection of disease changes compared to conventional methods
- Provide insight into therapy effectiveness, including chemotherapy, immunotherapy, or targeted approaches
- Enable longitudinal tracking of tumour cell activity during follow-up or periods of remission
- Inform research into how treatment strategies might be adapted over time
It applies to most solid epithelial tumours (e.g. breast, prostate, colon, lung).
While still under investigation, CTC analysis is being studied across a broad range of solid epithelial tumours (e.g., breast, prostate, colon, lung). For example, in breast cancer research, investigators are evaluating whether routine CTC monitoring (e.g., every 3–6 months) may offer insights into recurrence risk and help address patient concerns about uncertainty between scans.
CLINICAL PERFORMANCE
Sentinel qCTC builds on the foundation of a validated method that has been described in more than 100 peer-reviewed publications and investigated in ~30 clinical trials. This body of research demonstrates the potential of CTC analysis for:
- Reliable detection of rare, single living tumour cells in blood
- Quantitative insights into minimal residual disease and overall disease burden
- Associations between increasing CTC counts and higher recurrence risk in research settings
Frontier has advanced this approach into Sentinel qCTC to support ongoing exploration of how living CTC measurements can contribute to a deeper understanding of cancer biology, treatment response, and disease dynamics.
To support researchers and clinicians in these studies, Sentinel qCTC generates reports that include clear, illustrated summaries of results, designed to be communicated in plain, accessible language.

QUANTUM FLUOSCOPE QF1
An AI-powered fluorescence microscope that brings cancer cell analysis directly into the hospital or lab – in real time.
HOW THE AI PLATFORM WORKS
The QF1 is a clinical-grade fluorescence microscope, developed by Frontier Diagnostics to analyse blood samples stained for tumour cells. The platform;
- Detects and takes images of CTCs with high sensitivity
- Uses AI algorithms to standardise cell counting and reduce subjectivity
- Generates objective, reproducible results clinicians can trust
- Differentiates using AI between living and dead cells for a real quantitative result
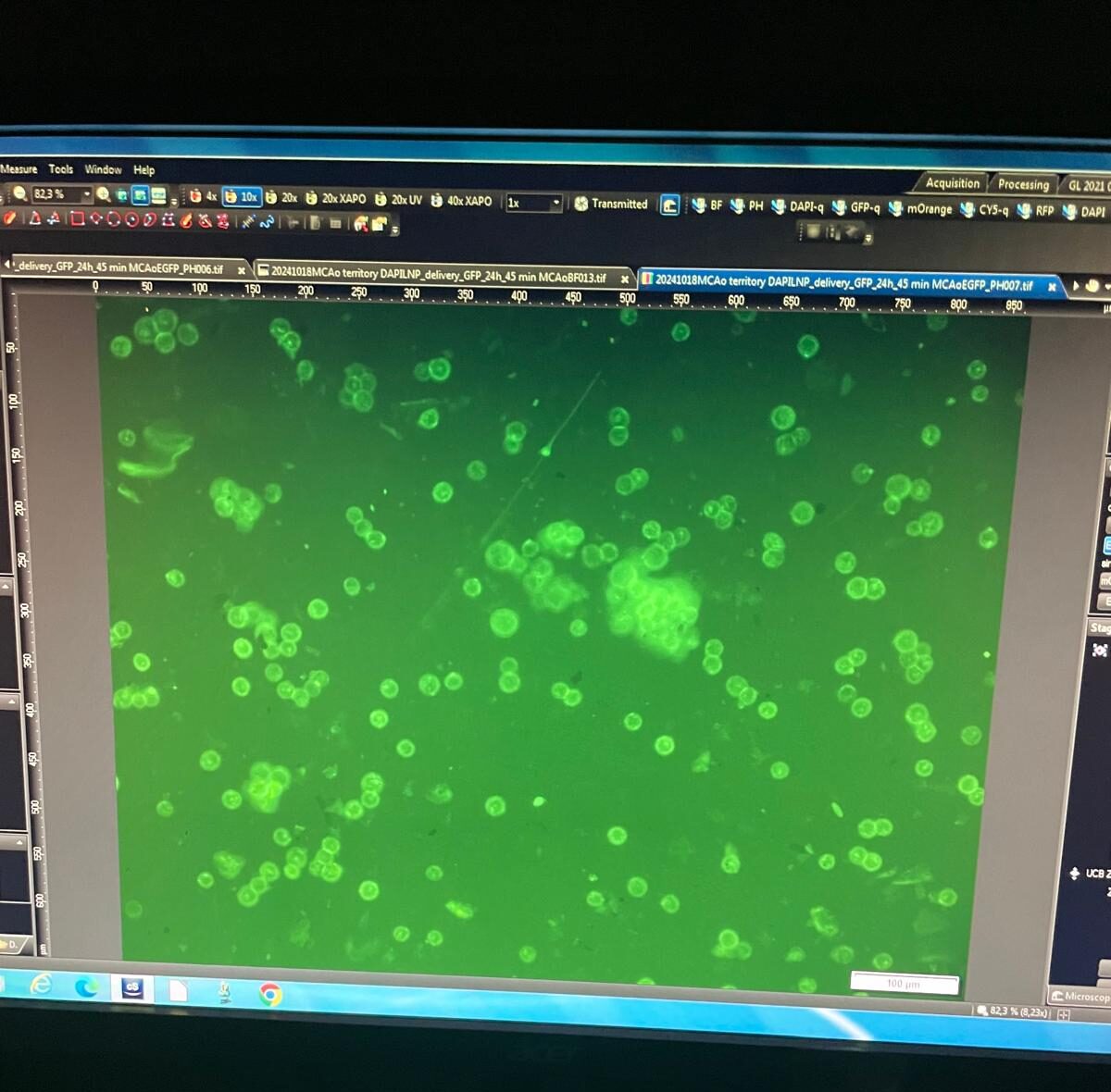
DESIGNED TO WORK SEAMLESSLY WITH SENTINEL qCTC
The Quantum FluoScope (QF1) has been developed to integrate directly with Sentinel qCTC, enabling laboratories and hospitals to perform liquid biopsy assays on-site. This design removes the need to ship samples to a central lab and reduces turnaround time from days to just a few hours.
- Labs and hospitals can perform liquid biopsy tests on-site
- Eliminates the need to ship samples to a central lab
- Cuts turnaround time from days to hours
By combining Sentinel qCTC with the Quantum FluoScope, researchers gain rapid access to results, supporting faster scientific insights and enabling studies that more closely reflect real-time tumour dynamics
BENEFITS FOR HEALTHCARE PROFESSIONALS & LABORATORIES
Sentinel qCTC has been developed to support modern research into circulating tumour cells with a platform designed for both sensitivity and efficiency. Key features include:
- Reliable detection of individual, living CTCs
- Throughput of up to 20 samples in under 3 hours (final evaluation stage)
- Seamless integration with LIMS and electronic record systems
- User-friendly workflow, designed with CE compliance in preparation
- Suitable for high-volume medical laboratories and research centres
By combining high analytical performance with operational efficiency, Sentinel qCTC enables laboratories and hospitals to expand their research (and soon medical diagnostics) capabilities in liquid biopsy — helping them explore new scientific frontiers while streamlining processes.
CLINICAL WORKFLOW
(SAMPLE-TO-RESULT PATH)
Simple, streamlined process from patient to report.
TEST COMPARISON CHART (FOR PATIENTS & HCPs)
Understand how Frontier Diagnostics tests differ from standard methods.
This helps patients and doctors choose the right monitoring mix.
| FEATURE | SENTINEL qCTC | STANDARD IMAGING (CT, MRI) | ctDNA |
|---|---|---|---|
| Invasiveness | Minimally invasive blood test | Radiation & scan; some discomfort | Not quantitative, only targeted mutations, dead cell measurement |
| Detects living tumour cells | Yes | No | No |
| Real-time monitoring | Yes | Limited | Close, however non quantitative |
| Repeatability | Easy & safe | Less practical | Expensive, non quantitative |
| Early detection | Far earlier than scans | Detects visible lesions only | Earlier than scans |
| Drug testing | Yes | No | No |
| Long-term monitoring | Yes | Yes, but late due to detection limits | Depends, only for certain mutations |
ORDERING & LOGISTICS
Easy access for patients and professionals.
Doctors or clinics order blood collection sets online: sets include a quick start guide and checklist as well as information booklets for both patients and doctors.
There is also a medical survey, questionnaire and lab request form in the test collection set which maintains blood samples at the right temperature.
GET IN TOUCH
Please fill in the form if you would like to be added to a waitlist to order our test kit.